Influenza A And B Test Kits

Cobas sars cov 2 influenza a b 09216235001 01en doc rev.
Influenza a and b test kits. Most of the rapid influenza diagnostic tests that can be done in a physician s office are approximately 50 70 sensitive for detecting influenza virus antigens and greater than 90 specific. Positive test for flu a influenza a antigen present flu a. There is no time like id now. Every year millions of americans see the doctor with flu like symptoms but only a small percentage will be positive.
The id now influenza a b 2 assay delivers molecular flu results in 13 minutes or less on our unique id now platform. This test is intended to be used by professionals particularly under the following circumstances. Display interpretation flu a. The results will be analysed statistically and the performance of the influenza a b test kit can be evaluated.
Nasopharyngeal swabs are tested using both the investigational test kit and a predicate fluorescence immunoassay test kit. All samples will be tested using a molecular based pcr test kit as a confirmatory test. Plus a new name. The fastest rapid molecular flu test.
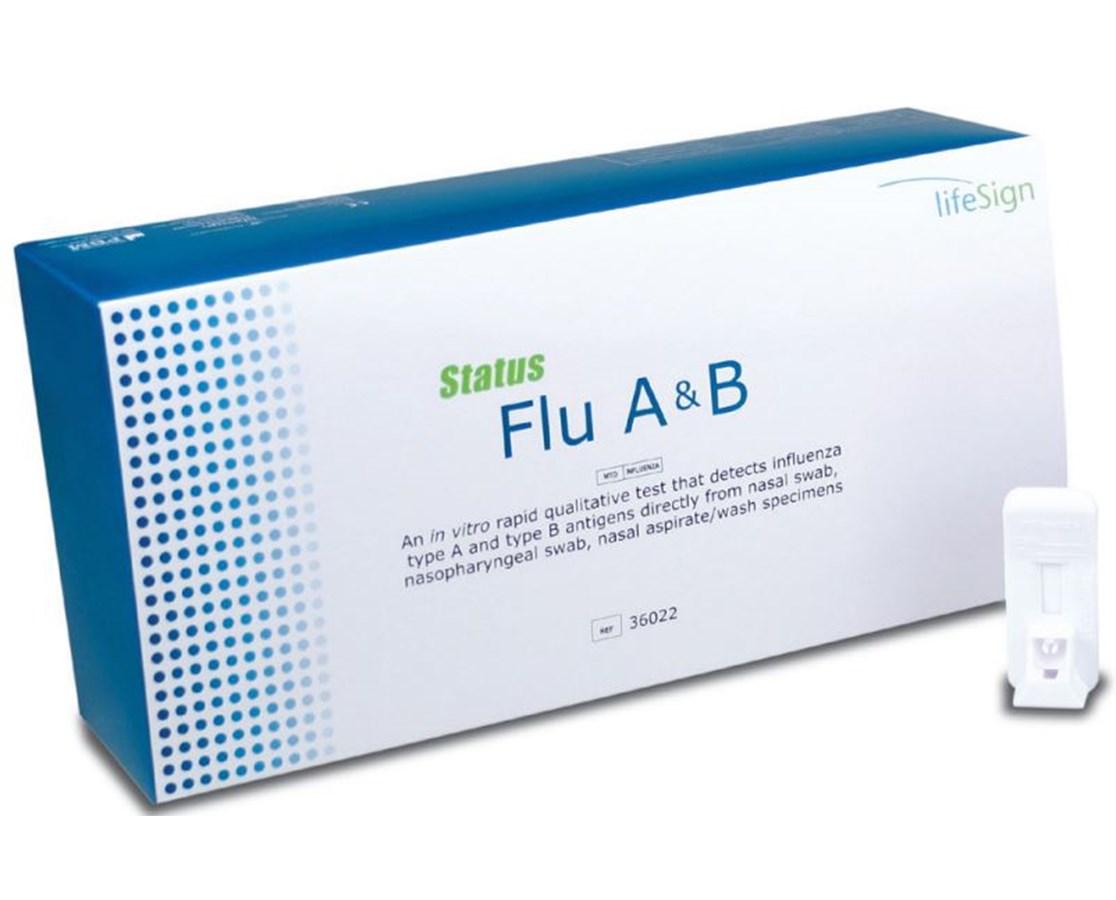
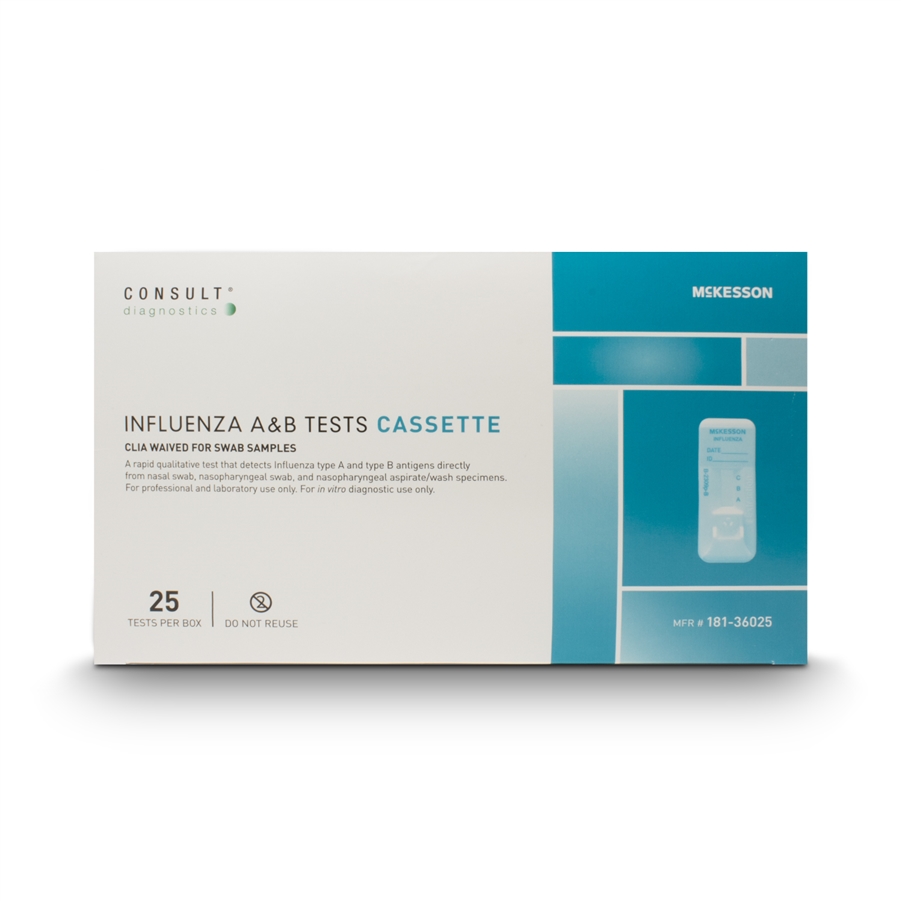
Benefits of a clia waived rapid flu test. A single sample can be used to run both the quickvue influenza a b test and quickvue rsv10 test. Mckesson consult influenza a b tests rapid qualitative test that detects influenza type a and type b antigens directly from nasal swab nasopharyngeal swab and nasopharyngeal aspirate wash specimens color coded control swab packaging for easy positive negative identification results in under 15 minutes. Fda cleared and clia waived.
1 0 7 cobas sars cov 2 influenza a b store at 2 8 c 20 tests p n 09211101190 20 transfer pipettes 1 package insert barcode card. The quickvue influenza a b test detects and differentiates influenza type a and type b antigens directly from nasal swab and nasopharyngeal swab specimens. The specificity and in particular the sensitivity of rapid influenza diagnostic tests are lower than for viral culture and rt pcr and vary by test. Good laboratory practice recommends using external controls positive and negative to assure the proper performance of the assay.
Making it significantly faster than other molecular methods and more accurate than conventional rapid tests. 25 tests quickvue influenza flu a b test kits dipstick format allows for the rapid qualitative detection of influenza type a and type b antigens directly from nasal swab nasopharyngeal swab nasal wash and or nasal aspirate specimens. Negative test for flu a and flu b. When the binax rapid flu test is performed with a nasal swab it will differentiate between influenza a and influenza b using one simple.
If the kit controls do not perform as expected do not report patient results and contact bd technical support at 1 800 638 8663. The positivia influenza a b rapid test external control kit is intended for use with the influenza a b rapid test as external assay controls to monitor test performance. Now available for immediate sale.