Influenza A And B Vaccine

The gov means it s official.
Influenza a and b vaccine. Helps prevent spreading flu to family and friends including babies younger than 6 months who are too young to get a flu vaccine. In the uk for the 2018 19 season. Recommended composition of influenza virus vaccines for use in the 2020 2021 northern hemisphere influenza season 28 february 2020. An h1n1 h3n2 and influenza b strain are included in the trivalent vaccine while an.
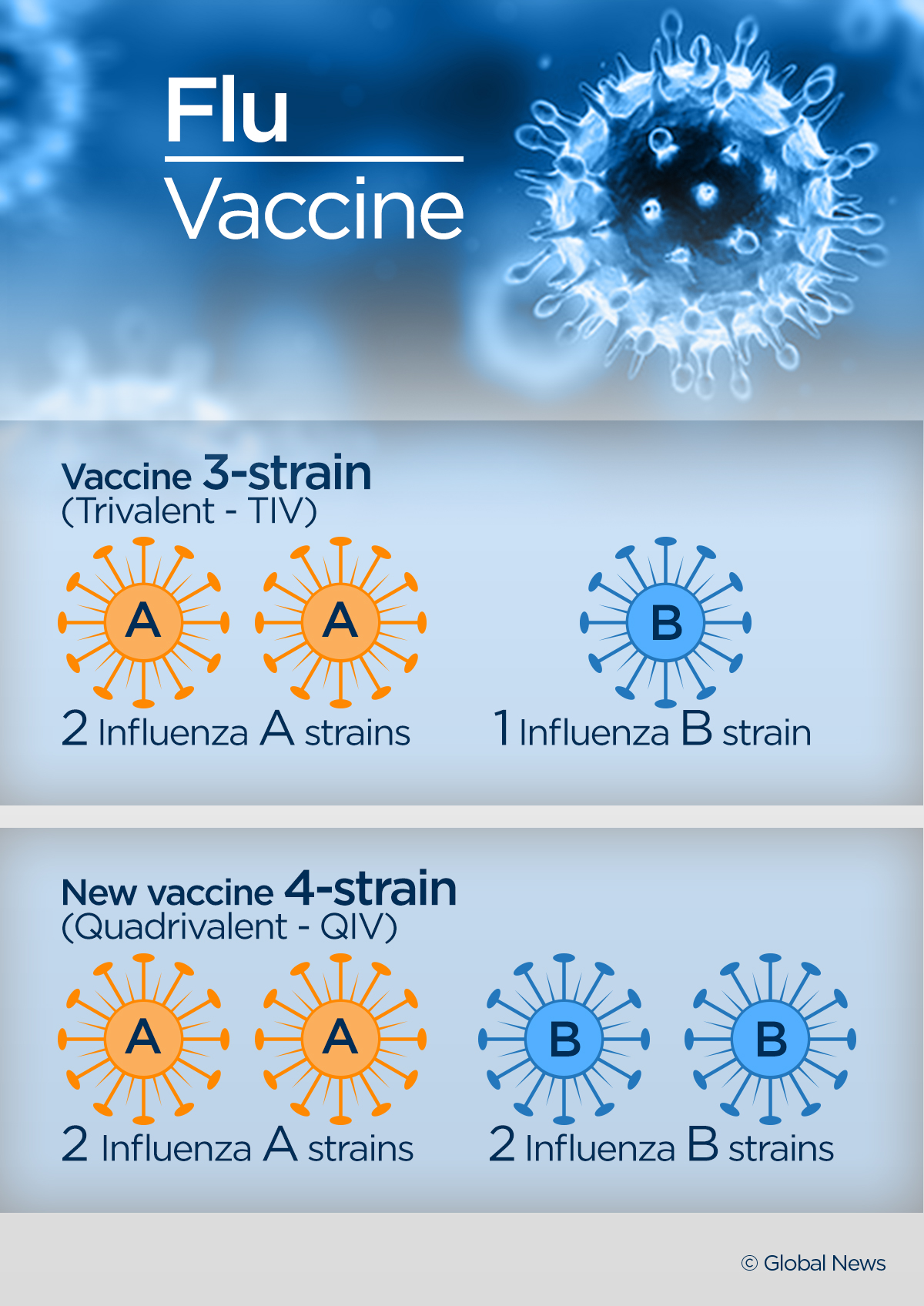
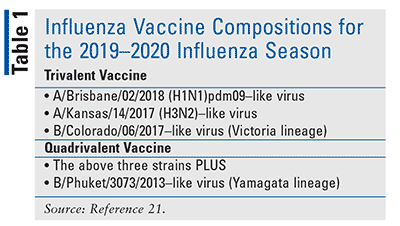
An a victoria 2570 2019 h1n1 pdm09 like virus. An a hong kong 2671 2019 h3n2 like virus. A b washington 02 2019 b victoria lineage like virus. For many years flu vaccines were designed to protect against three different flu viruses trivalent vaccines.
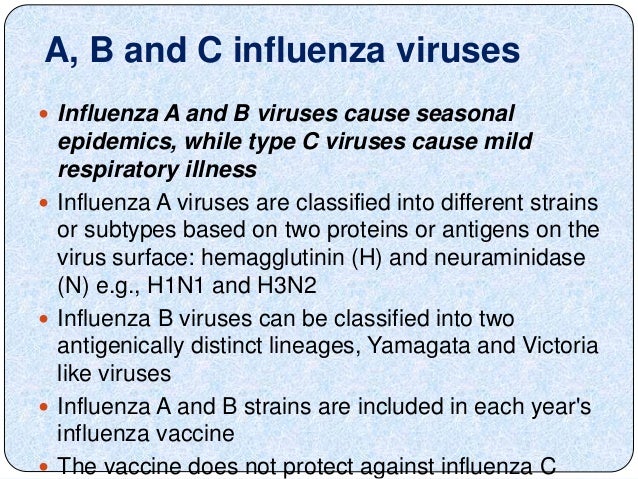
Each year the annual flu vaccine protects against three trivalent or four quadrivalent strains of flu. Before sharing sensitive information make sure you re on a federal government site. Trivalent vaccines protect against an influenza a h1n1 virus an influenza a h3n2 virus and one influenza b virus even though there are two different lineages of b viruses that both circulate during most seasons. For trivalent influenza vaccines for use in the us for the 2020 2021 influenza season depending on the manufacturing method of the vaccine the committee recommended that the a h1n1 pdm09 a h3n2 and b victoria lineage viruses recommended above for the quadrivalent vaccines be used.
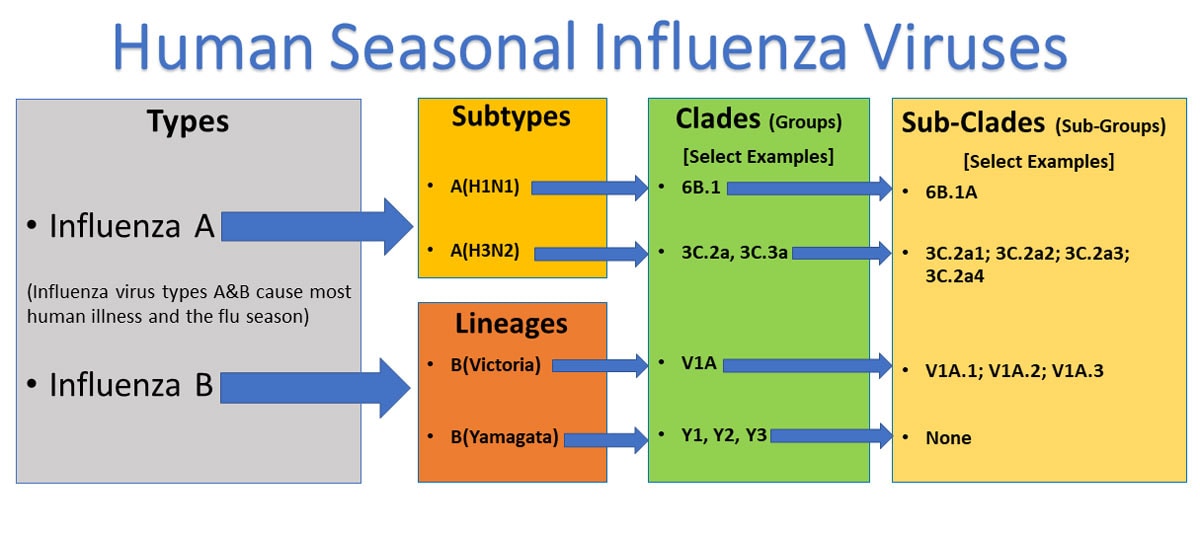
There are three types of flu viruses. A b and c. The adjuvanted trivalent vaccine ativ should be given to all aged 65 and over. Type a and b cause the annual influenza epidemics that have up to 20 of the population sniffling aching coughing and running high fevers.
Influenza a h1n1 2009 monovalent vaccine is an inactivated influenza virus vaccine indicated for active immunization of persons ages 6 months and older against influenza disease caused by pandemic h1n1 2009 virus. Reduces the high risk of developing serious flu complication especially if your child is younger than 5 years or of any age with certain chronic conditions. It is recommended that the influenza b virus component of trivalent vaccines for use in the 2018 2019 northern hemisphere influenza season be a b colorado 06 2017 like virus of the b victoria 2 87 lineage. This indication is based on the immune response elicited by the seasonal trivalent influenza virus vaccine manufactured by csl afluria.
A b phuket 3073 2013 b yamagata lineage like virus.